What Color is Positive on a Battery? (Guide)
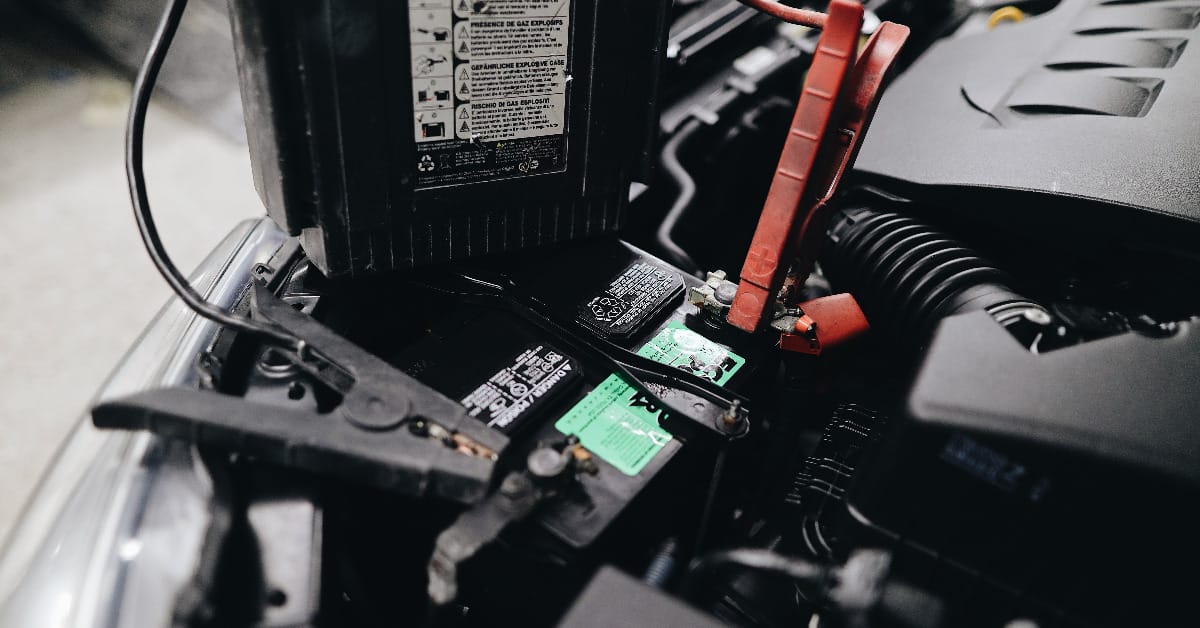
Let’s get a little dirty today with some car battery basics! I’m here to walk you through a simple yet crucial part of car maintenance – connecting those battery cables.
Key Takeaway: The positive terminal on your car battery is where the red cable belongs, while the black cable finds its home on the negative terminal. Sometimes, the terminals are color-coded, but other times, you’ve got to play detective and look for the ‘+’ and ‘-‘ signs.
But wait, there’s more to this electrical rodeo. We’re not just stopping at connecting cables. I will dive into some basic troubleshooting. Plus, I’ll share some handy maintenance tips to keep your car’s battery in peak condition.
The Science Behind the Spark
Think of a car battery as a silent powerhouse. It stores electrical energy and supplies it to the car when needed, especially to start the engine. Inside, it’s like a busy city at rush hour – electrons moving back and forth during discharge and recharge.
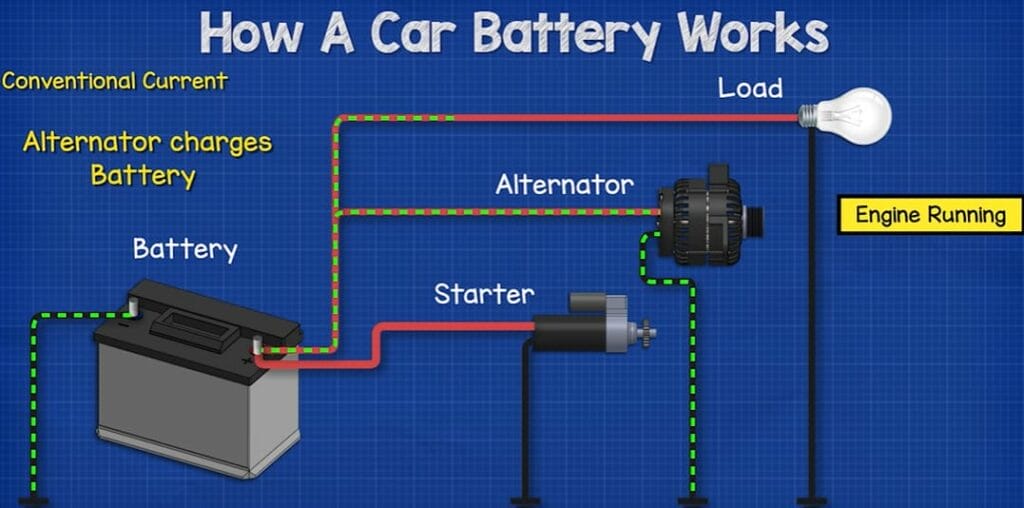
The Chemistry of Lead-Acid Batteries: These are the veterans in the battery world. They work on a simple yet fascinating chemical reaction. When you turn the key, sulfuric acid in the battery reacts with lead plates, creating lead sulfate and releasing electrons – that’s your car’s get-up-and-go energy. Recharging reverses this reaction, bringing the battery back to life.
Lithium-Ion Batteries – The Modern Players: These are the new kids on the block. They operate on a different principle. Lithium ions move between the anode and cathode, not electrons like in lead-acid batteries. This type of battery is lighter and can store more energy – think electric cars and high-tech gadgets.
There’s a difference between lead-acid and lithium-ion batteries. Lead-acid ones are heavier and have a lower energy density, but they’re simpler and more robust – great for traditional cars. Lithium-ion, however, can pack a lot of power in a small package, perfect for modern, energy-hungry devices.
Terminals of Car Batteries
Alright, DIY wizards, let’s amp up our knowledge with advanced technical details on car batteries. I’ve spent countless hours under the hood, and let me tell you, there’s a whole world of science powering these everyday wonders.

Positive and Negative: The Dynamic Duo
In my days of revamping and fixing, I’ve seen my fair share of batteries. Here’s the deal: your positive terminal is usually dressed in red and marked with a ‘+.’ That’s your red-wire hero.
The negative terminal? It’s the cool character in black, sporting a ‘-.’ This isn’t just random; it’s a universal code that keeps things straightforward and safe.
What’s Behind the Colors
| Terminal | Convention | Function |
|---|---|---|
| Positive | Red | Cathode |
| Negative | Black | Anode |
Now, let’s dive a bit deeper. The positive terminal is like the outgoing buddy – connected to the cathode. The cathode is made of lead oxide in those common lead-acid batteries I often tinker with. And in the world of lithium-ion batteries, it’s a mix of fancy stuff like nickel, manganese, and cobalt oxides.
On the flip side, the negative terminal, connected to the anode, is where the electrons enter. This is metallic lead in lead-acid batteries, and in lithium-ion ones, it’s usually graphite when you connect these two with a load in between; bam! You’ve got yourself a circuit.
Color Coding: More Than Looks
You might think color coding is just for show, but it’s a lifesaver. Imagine if you got those mixed up.
You’d be looking at a short circuit and much battery drama. I’ve seen it happen, and it’s not pretty.
Connecting the Dots: Red First, Then Black

Back to the basics – remember, red goes first. That red cable is your lifeline to the positive terminal. Then, the black cable follows, connecting to the negative terminal. This sequence is crucial. I’ve had my share of mix-ups, and trust me, you don’t want to learn this lesson the hard way.
I always keep a reminder in my toolkit: red for positive, black for negative. It’s simple but saves the day every time.
Let’s deep dive into some real-world scenarios with car batteries, spiced up with a bit of science and my own hands-on experiences. Trust me, these stories are not just about the nuts and bolts; they’re about understanding the why behind the what.
Effect of Connecting Wrongly

Imagine this – you accidentally swap the red and black cables. In the car battery world, this is a major no-no. Here’s the science behind it: Car batteries operate on a principle called ‘electrical polarity.’
Red is positive (+), loaded with a higher electrical potential, and black is negative (-), the grounding point. Swap these, and you disrupt the electrical flow. It’s like reversing the flow of a river – nothing flows smoothly.
I’ve been there, doing this by accident. The result? The car won’t start. Why? Because the electrical current is flowing in the wrong direction, confusing the car’s electrical system. It’s sending instructions in a language your car doesn’t understand.
Direct Terminal Connection – A Recipe for Disaster

Now, onto a more dramatic scenario – directly connecting both terminals. This one’s a bit of a science lesson in itself. Connecting positive and negative directly creates a ‘short circuit.’
It allows the electricity to flow unrestricted, creating a massive current surge. This can heat the battery rapidly, leading to potential damage or even a fire. It’s a real-life example of how powerful – and dangerous – uncontrolled electricity can be.
I recall a time I witnessed this happen. The battery got so hot that it was like a mini bonfire under the hood. That’s when I truly grasped the power of these seemingly simple car parts.
Unmarked Terminals – The Mystery and the Solution

But what if those terminals aren’t marked? This can turn into a real puzzle. Here’s a tip from my playbook – the positive terminal is generally larger than the negative.
Why? It’s all about reducing the risk of wrong connections. The design guides you to the right connection by making the terminals different sizes.
I remember scratching my head over an old battery with faded markings. The size difference was my savior. It’s a clever bit of engineering – simple but effective.
So, there you have it – a blend of science, safety, and real-life tales from car batteries. Remember, understanding the ‘why’ behind the ‘how’ can make you a better DIYer and a safer one too!
Hey everyone! You know, over the years, I’ve tackled all sorts of DIY challenges, and let me tell you, there’s nothing quite like the thrill of solving a tricky problem with a bit of know-how and elbow grease.
Today, I’m super excited to share a handy guide that I’ve put together based on my own experiences.
| Problem | Possible Cause | My Solution |
|---|---|---|
| The car won’t start after connecting the cables | Improper connection | Always double-check that the red cable is on the positive terminal and the black on the negative. Ensure they’re tight and secure. |
| The car starts but then dies | Weak or old battery | This takes me back to a project where the car would start but couldn’t hold the charge. It turned out the battery was just too old to function properly. It might be time for a battery replacement. |
| Clicking sound, but the car won’t start | Dead battery or bad connection | Ah, the classic clicking sound! I’ve had this happen a couple of times. Usually, it’s either a dead battery or a loose connection. Tighten those cables, or you might need to jump-start the battery. |
| Electrical components not working | A blown fuse or battery issue | It reminds me of a situation where the radio and lights wouldn’t work. Check for a blown fuse first. If the fuses are fine, then it’s likely a battery issue. |
| The car starts with a jump but dies soon after | Alternator issues | This can be tricky. I faced this once, and it turned out to be the alternator, not the battery. If your car dies after a jump, it might be time to check the alternator. |
Frequently Asked Questions
- How Do I Know If My Battery Is Fully Charged?
- This one’s a bit technical, but you can use a multimeter to check the voltage. A fully charged car battery should read around 12.6 volts or higher. I always keep a multimeter in my toolkit for quick checks.
- Can Jumpstarting Damage My Car?
- If done correctly, jumpstarting is generally safe. But, if you reverse the cable connections or connect a live battery to a dead one, there’s a risk of damage. Always follow the proper jumpstarting procedure. Trust me, it saves a lot of headaches.
- Can a Car Battery Recharge Itself After Being Dead?
- This is a common misconception. A dead battery can’t recharge itself. You might need a jump start or a battery charge. Remember, regular driving recharges the battery, but it won’t return from the dead without a little help.
- Why Does My Battery Die If I Leave the Lights On?
- Ah, the classic left-the-lights-on scenario! Your battery powers all electrical components in the car. If the engine’s off and the lights are on, the battery drains without being recharged by the alternator. I’ve made this mistake several times, and it’s always a facepalm moment.
- Do I Need to Add Water to My Car Battery?
- Modern car batteries are usually maintenance-free, meaning you don’t need to add water. But, if you have an older style (non-sealed) battery, you might need to check and occasionally top up the water level. Always use distilled water for this.
- Can Extreme Temperatures Affect My Car Battery?
- Absolutely! Extreme cold can slow down the chemical reaction inside the battery, making it harder to start your car. Extreme heat, on the other hand, can speed up battery corrosion. I always advise checking your battery before the start of winter or summer.
References
Organizations:
- Battery Council International (BCI). https://batterycouncil.org/
- The Electrochemical Society. https://www.electrochem.org
Books:
- “The Battery: How Portable Power Sparked a Technological Revolution” by Henry Schlesinger. https://www.barnesandnoble.com/w/battery-henry-schlesinger/1101057504
- “Battery Management Systems for Large Lithium-Ion Battery Packs” by Davide Andrea. https://www.barnesandnoble.com/w/battery-management-systems-for-large-lithium-battery-packs-davide-andrea/1022678418
Website Resources:
- Battery University. https://batteryuniversity.com/
- AA1Car Automotive Diagnostic Help Center. https://www.aa1car.com
Video References:
The Engineering Mindset
Stillwater Hardy
World Mechanics
briansmobile1
ProjectDIY
