Does Sulfuric Acid Conduct Electricity?

Sulfuric acid is a chemical found in many homes and businesses. Does it conduct electricity? Does greater concentration make a difference to its electrical conductivity? What is sulfuric acid used for if it does conduct electricity? Before explaining in detail, here’s the short answer:
Yes, sulfuric acid conducts electricity very well. It has special uses due to its high electrical conductivity. However, it is highly corrosive, so it must be used cautiously.
Beware! Sulfuric acid is a highly corrosive substance. It is destructive when it comes into contact with the skin or eyes or if inhaled. Severe exposure to it can even lead to death. Handle it very carefully.
What Makes Sulfuric Acid Conduct Electricity?
Autoprotolysis and Ionization
Sulfuric acid, a mineral acid with the chemical formula H2SO4, contains hydrogen, oxygen, and sulfur. It is a colorless, odorless, viscous liquid miscible with water. Sulfuric acid’s ability to conduct electricity well is due to autoprotolysis. This is a chemical reaction in which protonation (proton transfer) occurs between identical molecules, allowing for dissociation.
When sulfuric acid is dissolved in water, the solution ionizes by separating into hydrogen (H3O+) and sulfate (HSO4–) ions. It is these ions that carry charges and enable them to conduct electricity. When added to water, it makes sulfuric acid an even better conductor of electricity, which makes it very useful in many ways. Before we explore them, let’s see what difference the concentration makes to how well sulfuric acid conducts electricity.
Does Greater Concentration Make Sulfuric Acid More Electrically Conductive?
Diluted sulfuric acid has less than 30% of sulfuric acid content by mass, and concentrated sulfuric acid has over 98%. You might think that concentrated sulfuric acid would be a better conductor of electricity than a dilute form, but this is not true.
Concentrated sulfuric acid has lower electrical conductivity than if diluted. This is because of fewer H+ and SO42- ions in the concentrated form. The greater concentration makes it denser than diluted sulfuric acid but reduces its electrical conductance. Diluted sulfuric acid is more electrically conductive because of the larger H+ ions.
Uses of Sulfuric Acid as a Conductor
Safety Precautions First
Safety precautions are essential when handling anything that contains sulfuric acid because it is hazardous and highly corrosive. It can cause very severe burns, especially if highly concentrated. It is, therefore, critical to wear the following protective equipment:
- Wear hand protection, such as gloves.
- Wear a safety apron.
- Wear goggles or put on a face visor.
Widespread Uses
Sulfuric acid has many uses. For example, it is used in homes as a drain or toilet bowl cleaner. In the chemical industry, it produces glue, detergents, insecticides, and other chemicals; in the military, it makes explosives. It also uses agriculture, paint, printing, automobile, and other industries. It is a very important commodity.
Most of these uses are related to cleaning, dehydrating, or oxidizing. However, sulfuric acid is also very useful due to its electrical properties. This is explored in detail next.
Sulfuric Acid Used as an Electrolyte
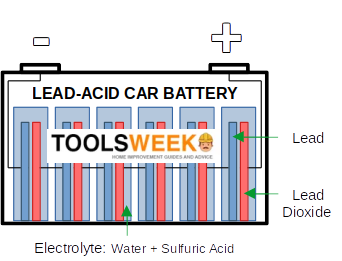
One of its most common uses for its electrical properties is in cars and other vehicle lead-acid batteries. In a lead-acid battery, sulfuric acid is used as the electrolyte in a car battery when mixed with water. In this way, not only does it conduct electricity, but it is also capable of storing electrical charge.
As long as a charging voltage is applied to the lead-acid battery, it separates into opposite pairs of ions, i.e., positive and negative. The ions are compelled to separate while the current flows into its positive terminal. In a fully charged state, the electrolyte solution (see picture below) has a high sulfuric acid concentration in liquid form. This stores most of the chemical energy. The battery then discharges while connected to a load. A lead-acid battery helps to start a vehicle with a combustion engine.
Wrapping Up
Does sulfuric acid conduct electricity or not? We explained that it does so very well. We showed that this is due to autoprotolysis. We explained how it can conduct electricity due to the ionization of hydrogen and sulfate ions and that less concentration in water makes sulfuric acid more electrically conductive. Furthermore, we described how sulfuric acid is used as an electrolyte in lead-acid batteries.
