Is Silicon a Good Conductor of Electricity?
Silicon is a semi-metal that conducts electricity, although pure silicon doesn’t. But what about its relative electrical conductivity, i.e., how good of a conductor of electricity is it? Before explaining in detail, here’s the short answer:
Silicon is a semiconductor that conducts electricity beyond a temperature adequately high to free its electrons. Otherwise, it can also function as an insulator. Doping (introducing impurities into its structure) can also alter its conducting properties. Its electrical conductivity (at 20°C) is 1.56×10−3 S/m, which rises with increasing temperature. It has many uses and is useful as a semiconductor in electronics.
I will tell you what silicon is, explain its electrical properties in detail, and explain why the electrical conductance of silicon matters to determine how well silicon conducts electricity below.
Silicon’s Electrical Conductance
Silicon’s electrical conductivity is 1.56×10−3 S/m at room temperature (20°C).
Its conductivity varies with temperature, as explained below.
How Well Silicon Conducts Electricity
What Makes Silicon a Good Conductor?
Pure silicon doesn’t conduct electricity well at all.
It has poor conductivity because its outer electrons form covalent bonds. For this reason, silicon is also used as an insulator and a conductor. As an insulator, it is useful for lowering power consumption and reducing junction capacity.
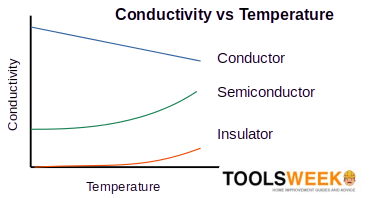
The dual insulating and conductive properties of silicon are because it’s a semiconductor (see the graph below). At low temperatures, it’s an insulator because it has no free electrons. Its free electrons flow to produce electric current at normal room temperature.
Silicon’s conductivity increases with increasing temperature, making it a conductor. The greater molecular vibrations due to greater heat energy free its electrons. This behavior is peculiar to semiconductors. It differs from metals, whose electrical conductance generally decreases with increased temperature.
The Usefulness of Silicon’s Electrical Conductance
Silicon has many uses.
For example, ceramics use it to make molds, opal rings, aerogel, etc. It is also added to steel to make it more magnetic and to make firefighting suits. It is particularly useful because it can react with both metals and non-metals. [Jackson, 2017]
Uses of Silicon in Electronics
An important area of use for silicon is electronics.
Here are a few important uses of silicon (due to its electrical properties):
- Silicon wafers and chips drive electronic circuits.
- It is used in solar panels to turn sunlight into electricity.
- It is used in making semiconductors. Silicon is a very versatile material in space semiconductor devices.
- It makes various electronic components, including diodes, transistors, and integrated circuits.
Usually, some impurities are added to silicon to give it the desired properties. This process is called doping; the impurity is often antimony or boron.
Gallium arsenide has similar and superior properties, but silicon offers a more widely available and cheaper alternative. [Gibilisco]
Silicon Deep Dive
Appearance and Properties
In crystalline form, silicon is gray, whereas amorphous silicon is a brown powder.
Predominance
Silicon is a chief constituent of many soils, clays, and rocks.
It is the second-most abundant element in our planet’s crust [Jackson, 2017]. It is also manufactured by reducing silica with carbon in an electric furnace. It is used in making certain alloys and glass. Its semiconductive properties make it useful in a large range of electronic components. [Chambers, 1991]
Chemical Classification
Silicon is a non-metallic element with the chemical symbol Si.
The name is derived from the Latin word silex, which means flint. Its valence number is 4. Berzelius discovered it in 1823.
References
Tom Jackson. The periodic table book: A visual encyclopedia of the elements. Dorling Kindersley. 2017.
Chambers. Chambers science and technology dictionary. Chambers. 1991.
Silicon Wafer: https://www.waferworld.com/post/silicon-wafer-processing-process.
Stan Gibilisco. Teach yourself electricity and electronics. Third edition. TAB Electronics. McGraw-Hill.
