Can Sugar Conduct Electricity?

When you imagine a material that can carry electricity, sugar is usually not the first thing that springs to mind, but the truth could surprise you.
Sugar is used in many food products, including cakes and chocolates. It creates a sugar solution in water and dissociates readily. But many remain unsure whether sugar solution transmits electricity or not, although we all know that electrolytic solutions like NaCl aqueous solution do. As an experienced electrician who loves chemistry, I will address this question and related subjects in this guide.
Quick Summary: Sugar solution doesn’t conduct electricity. The free ions needed to carry electricity are absent from the sugar solution. Covalent bonds hold sugar molecules together, preventing them from dissociating from free ions in water. Since it does not dissolve free ions as an electrolytic solution does, a sugar solution acts as an insulator.
I will do an in-depth analysis below.
Can Sugar Transmit Electrical Current?
The answer is NO, sugar solution doesn’t conduct electricity.
Reason: The free ions needed to carry electricity are absent from the sugar solution. Covalent bonds hold sugar molecules, so they don’t dissociate from mobile ions in water. The sugar solution is an insulator because, unlike an electrolytic solution, it does not dissociate free ions.
The Chemistry of Sugar Molecule
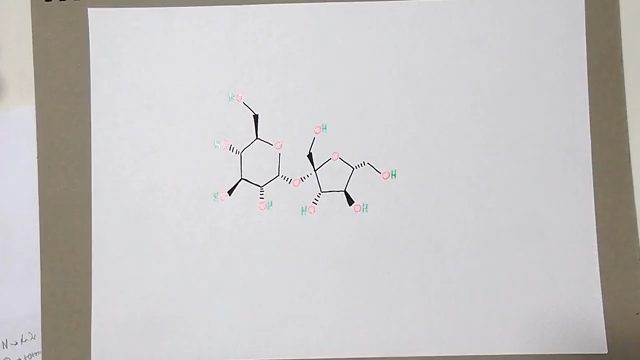
Formula: C12H22O11
12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms constitute the organic molecule known as sugar. Sugar has the chemical formula: C12H22O11. It’s also referred to as sucrose.
The compound sugars sucrose, lactose, and maltose share a chemical formula — C12H22O11
The one chemical that’s called sugar is sucrose. Sugarcane is the most common source of sucrose.
Bonding Type — Covalent
Covalent bonds bind carbon (C), hydrogen (H), and oxygen (O) atoms.
Aqueous Sugar — Are There Any Free Ions?
A sugar solution is obtained when sugar is introduced to (H2O) water and thoroughly stirred. Sugar and water molecules both contain hydroxyl groups (-OH). Thus, hydrogen bonds link the sugar molecules.
The sugar molecules do not dissociate, hence no covalent bond in the sugar molecules is broken. And only new hydrogen bonds between the molecules and the water are generated.
As a result, there are no electron transfers between the sugar molecules. Each electron remains tethered to its respective molecular structure. As a result, the sugar solution doesn’t contain any free ions that could conduct electricity.
Does Sugar Conduct Electricity in Water?
The electrolyte in an electrolytic solution, like NaCl and KCl, contains ionic bonding. They quickly dissolve into free mobile ions when added to (H2O) water, allowing them to travel throughout a solution and conduct an electrical current.
While sugar molecules are neutral, electrolytes are charged.
Solid State Sugar — Does it Conduct Electricity?
The carbon, hydrogen, and oxygen atoms in sugar, which have the chemical formula C12H22O11, are joined by covalent bonds as aforementioned.
- Because sugar molecules are neutral, if we place an electric voltage across a sugar crystal (solid), electrons do not travel across it. Covalent bonds also have an equal distribution of charges among the two atoms.
- The electron stays static and the sugar molecule functions as an insulator since the connection is non-polar.
- Free ions that serve as electricity carriers are necessary to pass electric current. It’s not possible to convey an electric current through a chemical complex without mobile ions.
Any chemical that can dissolve or dissociate in water without giving off ions is known as a non-electrolyte. Electricity cannot be conducted by a nonelectrolyte material in an aqueous solution.
Video Reference
How To For You
