Does Lithium Conduct Electricity?
Lithium is widely used in batteries and various electronics. It’s an alkali metal with distinctive characteristics in group one on the periodic table.
As an electrician who needs to know this for a living, I will share a few useful insights on lithium’s conductivity in this guide. Due to lithium’s vast industrial application, understanding its “chemistry” gives you an upper hand in its applications.
Quick Summary: Lithium conducts electrical current in solid and molten states. Lithium has metallic bonding, and its valence electrons are delocalized in the liquid and solid states, allowing electrical energy to flow. So, in a nutshell, lithium’s electrical conductivity solely relies on the availability of delocalized electrons.
I will capture more detail below.
Why Does Lithium Conduct Electricity in Molten and Solid States?
Presence of delocalized electrons.
Lithium has metallic bonding, and its valence electrons are delocalized in the liquid and solid states, allowing electrical energy to flow. So, briefly, lithium’s electrical conductivity solely relies on the availability of delocalized electrons.
Does Lithium Oxide Conduct Electricity in Both Molten and Solid States?
Lithium oxide (Li2O) only conducts electricity when it is molten. It is an ionic compound, and the ions in solid Li2O are localized in place in an ionic lattice; the ions are not free/mobile and thus cannot conduct electricity. When molten, however, the ionic bonds are disengaged, and the ions become free, allowing a seamless flow of electrical energy.
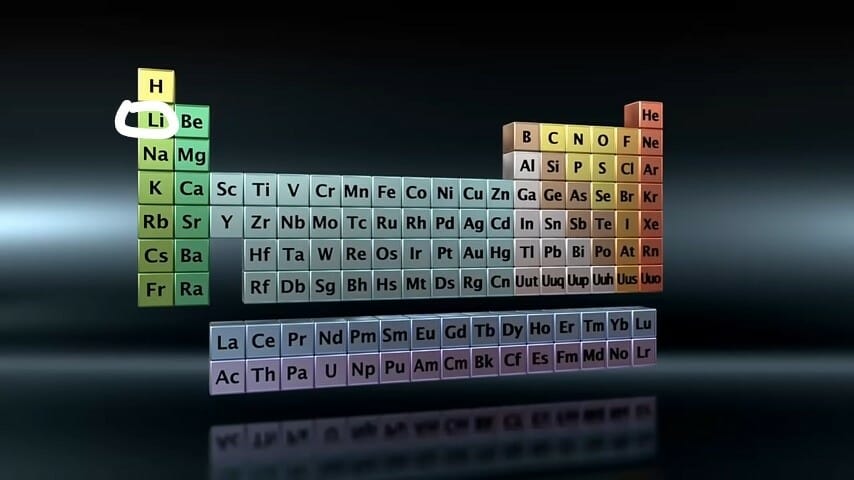
Where is Lithium on the Periodic Table?
Lithium is an alkali metal and is found in group one on the periodic table:
Its precise location is shown in the image below.
The Properties of Lithium, Li — Chemical and Physical
1. Atomic Number, Z
Lithium is a chemical substance with the atomic number (Z) 3, i.e. Z = 3. That translates to three protons and three electrons in its atomic structure.
2. Chemical Symbol
Lithium’s chemical symbol is Li.
3. Physical Appearance
It is a soft, silvery-white alkali metal that is the lightest. Also, it’s the lightest solid element under normal conditions.
4. Reactivity and Storage
Lithium (like all alkali metals) is extremely reactive and flammable and hence stored in mineral oil.
5. Atomic Mass, A
Atomic mass = 6.941 u.
The mass of an atom (lithium, in our case) is defined as its atomic mass. The atomic mass, also known as relative isotopic mass, refers to the mass of a single particle and is thus associated with a specific isotope of an element.
6. Boiling and Melting Point
- Melting point, MP = 180.5°C
- Boiling point, BP = 1342°C
It should be noted that these points are related to the standard atmospheric pressure.
7. Lithium Atomic Radius
Lithium atoms have an atomic radius of 128pm (covalent radius).
It should be noted that atoms lack a clearly defined outer boundary. The atomic radius of a chemical substance is the distance the electron cloud reaches from the nucleus.
Fun Facts about Lithium, Li
- Lithium is used in medicine, as a heat transfer agent, to make alloys, and in batteries, among other things.
- Even though lithium substances improve mood, scientists are still unsure how they work on the nervous system. What is known is that lithium reduces the action of the dopamine receptor. Also, it can cross the placenta to affect the unborn child.
- The first man-made nuclear fusion reaction was the conversion of lithium to tritium.
- Lithium comes from the Greek word lithos, which means “stone.” Lithium is found in most igneous rocks, but it is not found free.
- Electrolysis of (fused) lithium chloride (LiCl) produces lithium metal.
Working Principle(s) of Lithium-ion Battery
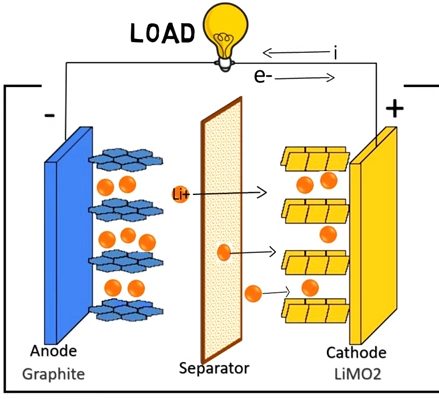
A rechargeable lithium-ion battery comprises one or more power-generating compartments known as cells. Each cell/compartment contains three main parts:
The Positive electrode (graphite) — is connected to the battery’s positive.
The Negative electrode — is connected to the negative.
Electrolyte — sandwiched between the two electrodes.
The movement of ions (via the electrolyte) and electrons (via the external circuit, in the opposite direction) are all interconnected processes; when one stops, the other follows.
If ions can’t flow through the electrolyte when the battery has completely discharged, electrons cannot either.
Similarly, if you turn off whatever the battery is powering, the movement of electrons and ions ceases. The battery effectively stops discharging rapidly — but it keeps discharging very slowly, even with an appliance disconnected.
Video References
AtomicSchool
Owl WiS
